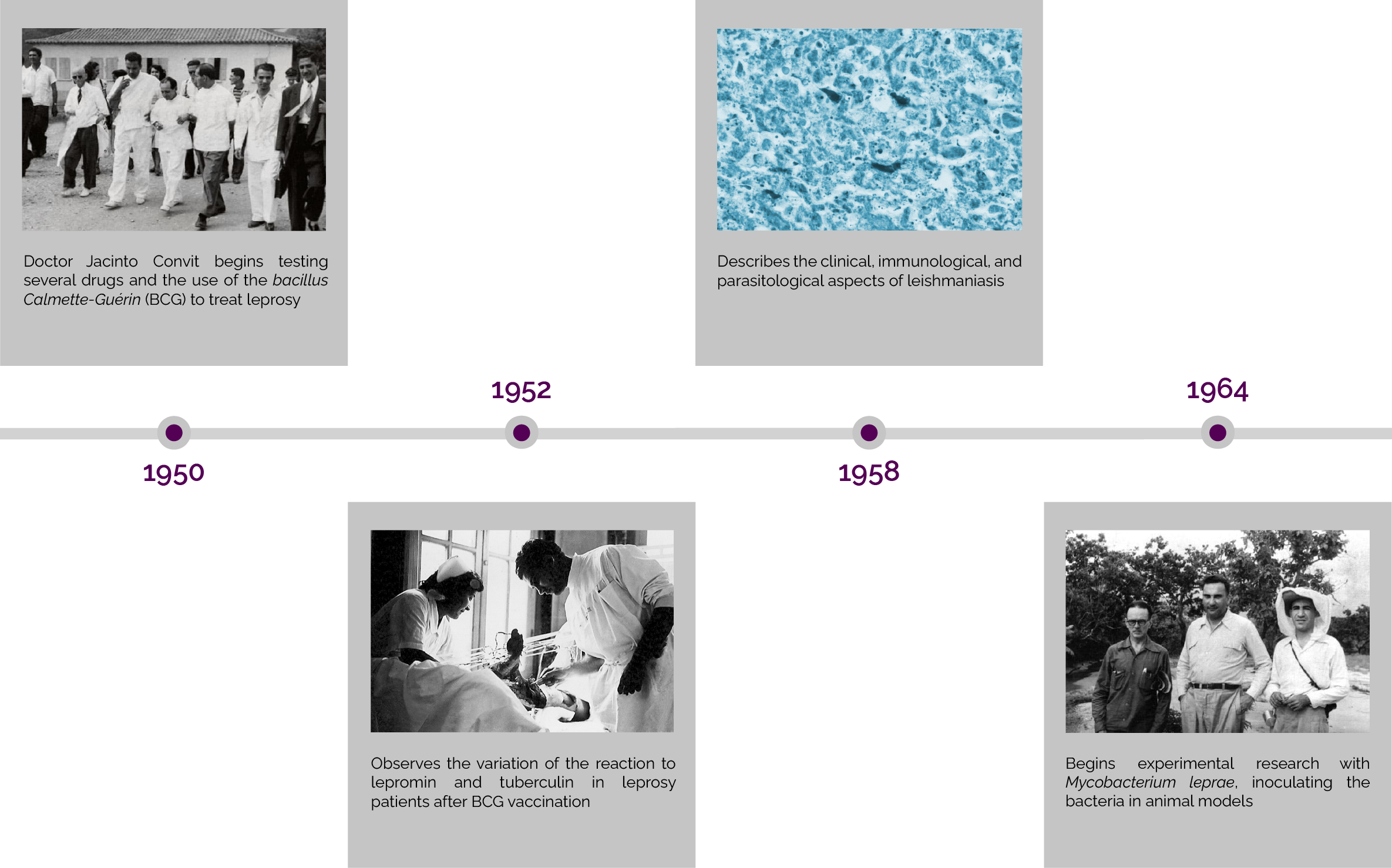
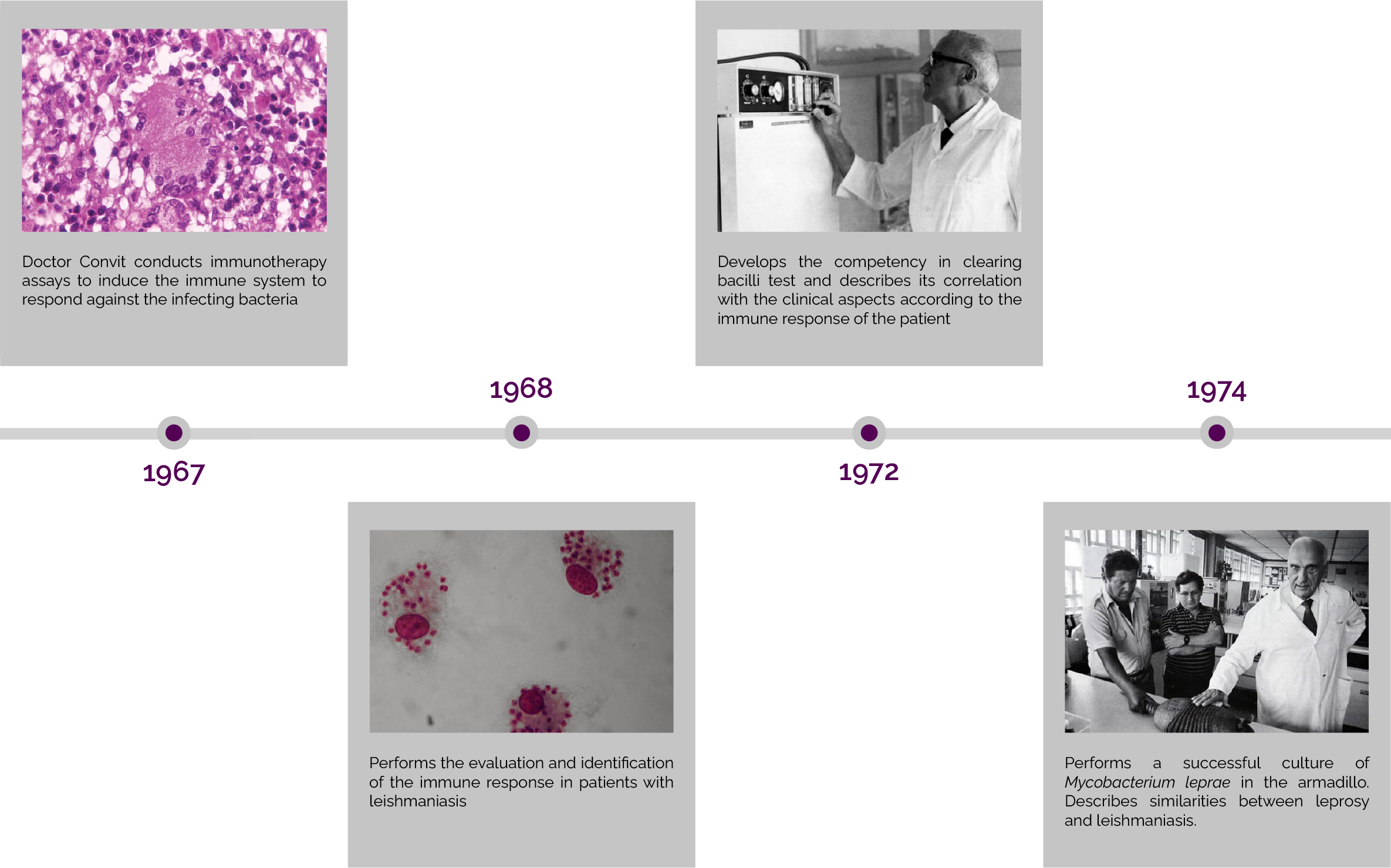
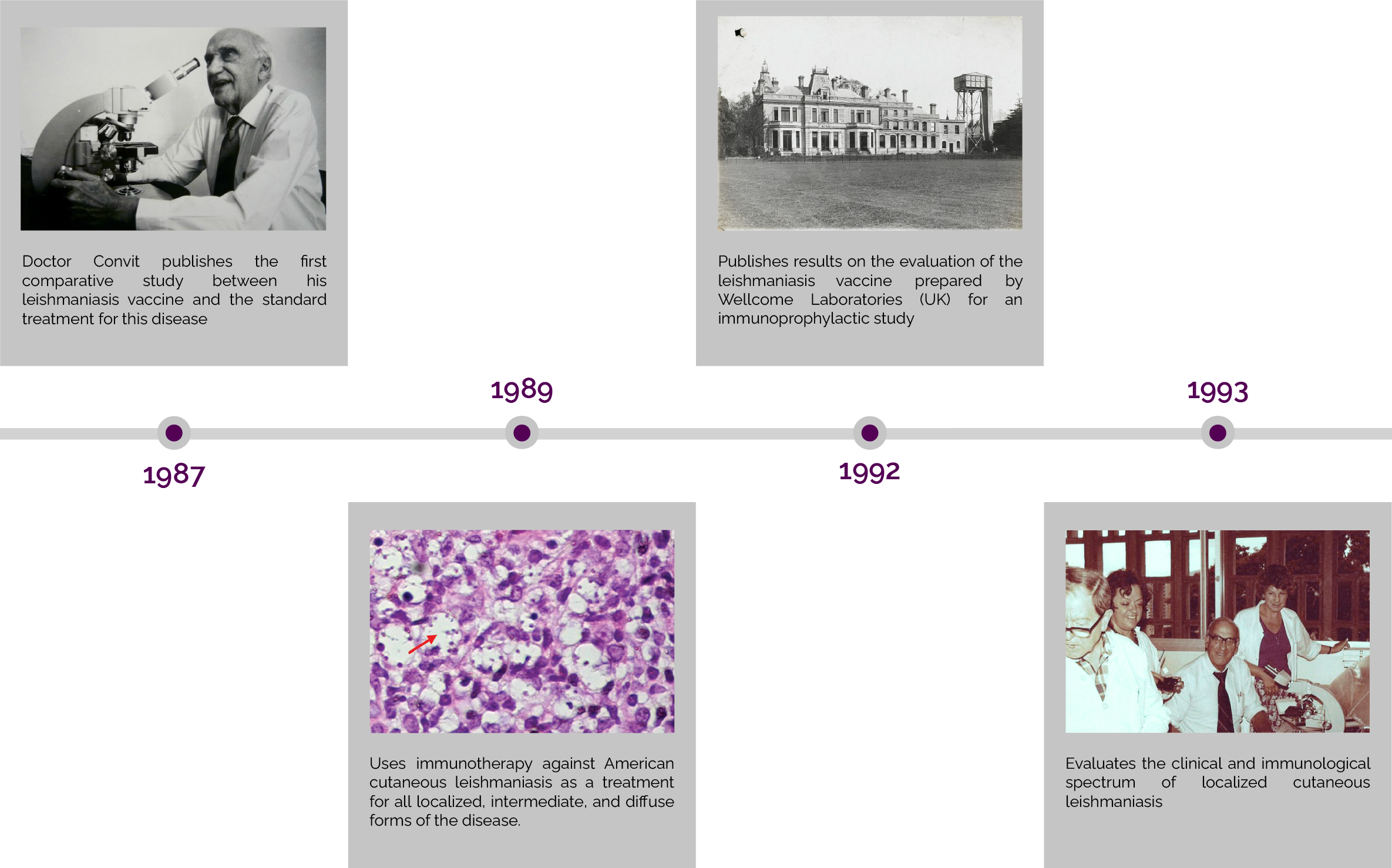
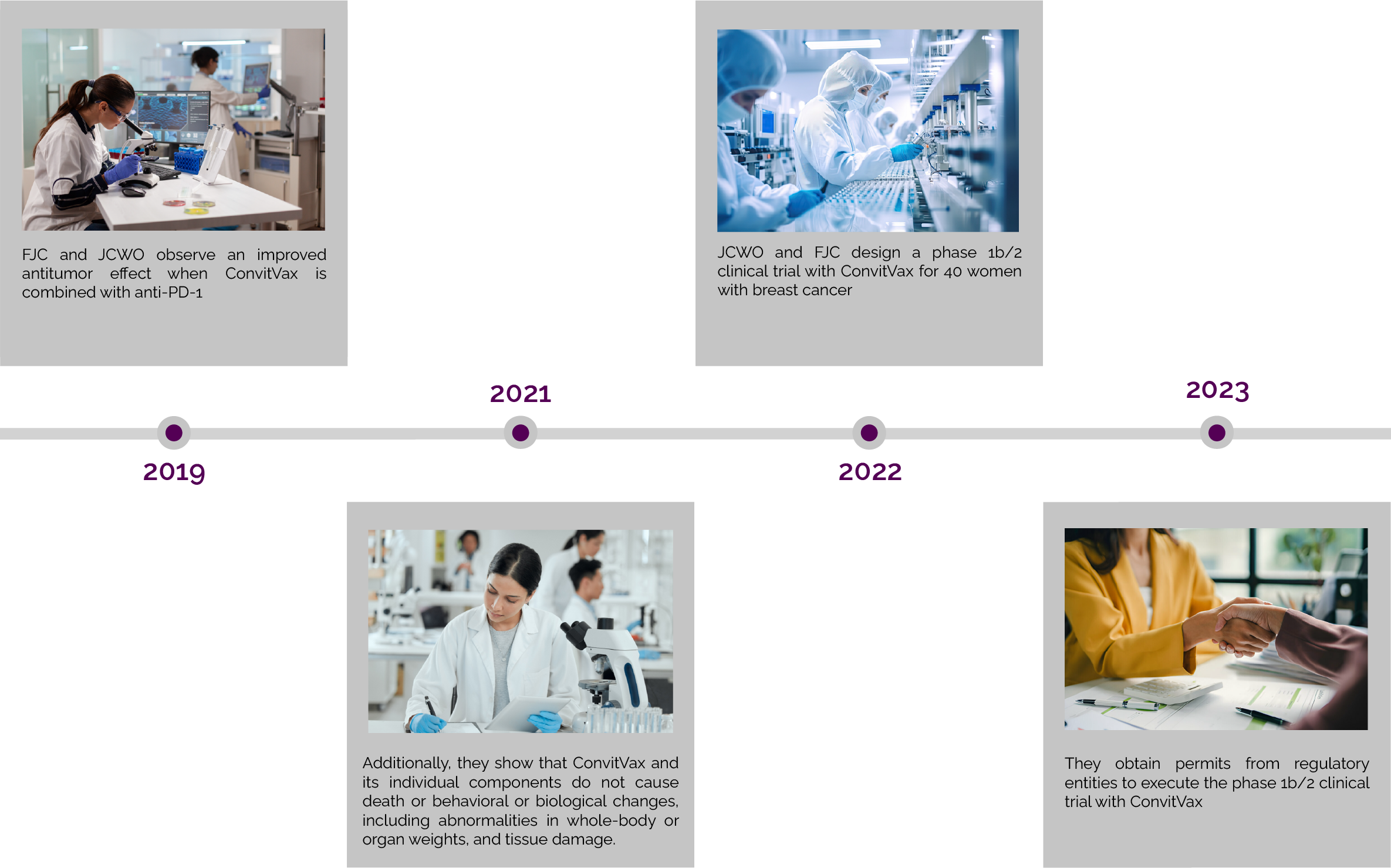
Cancer Immunotherapy
This program develops a low cost, simple, personalized, potentially safe and effective immunotherapy, named ConvitVax, targeted to underprivileged patients with breast cancer. This therapy combines the patient’s tumor cells with bacillus Calmette Guérin (BCG) and low concentrations of formalin. Studies in animals and a human pilot experience show that the combination of these components stimulate the immune system, achieving an effective and specific response against the body’s tumor cells with minimal to no side-effects and a possible establishment of immune memory, which may reduce the possibility of disease recurrence. JCWO seeks to build international partnerships and raise funds to carry out clinical studies with ConvitVax and continue further research on combination therapies.
Therapy development and FDA approval process

If you think you can contribute to this cause in any way
Our goal is to make ConvitVax accessible to underprivileged woman who lack access to expensive treatments. Walk through history to learn about the background of this therapy.